Discover What Color Red Litmus Paper Turns In Alkali
When red litmus paper is placed in an alkali, it turns blue. This color change is a simple yet fascinating indication of the solution’s basic nature. Alkalis have the ability to neutralize acids, resulting in the distinct transformation of red litmus paper to blue. Understanding this color change provides valuable insight into the properties of different substances and their chemical interactions. Discover more about this intriguing phenomenon as we delve into the world of red litmus paper and alkalis.
What Color Does Red Litmus Paper Turn When Placed in an Alkali?
The Science Behind Litmus Paper
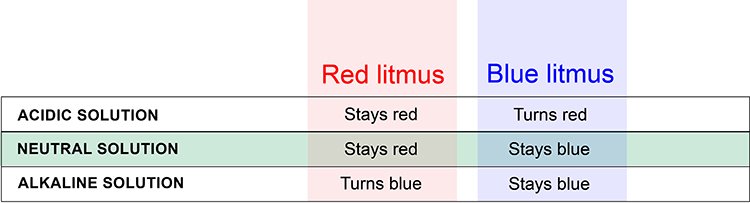
Litmus paper is a simple yet powerful tool used in chemistry to determine the acidity or basicity of a substance. It comes in two main types: red litmus paper and blue litmus paper. Red litmus paper is red in its neutral state, whereas blue litmus paper is blue. When either type of litmus paper comes into contact with an acidic or basic solution, it undergoes a chemical reaction that causes it to change color, providing valuable information about the nature of the substance being tested.
Understanding Acids and Bases
Before we dive into what happens when red litmus paper encounters an alkali, let’s briefly discuss acids and bases. Acids are substances that donate protons in a chemical reaction, while bases are substances that accept protons. Acids have a sour taste, can turn litmus paper red, and have a pH below 7. Bases, on the other hand, have a bitter taste, turn litmus paper blue, and have a pH above 7.
What Happens to Red Litmus Paper in an Alkali?
Now, let’s answer the burning question: what color does red litmus paper turn when placed in an alkali? When red litmus paper comes into contact with an alkali, such as sodium hydroxide (NaOH) or potassium hydroxide (KOH), a fascinating transformation takes place. The red litmus paper, which was red due to its acidic nature, reacts with the alkali and changes color.
The Color Change
The color change that red litmus paper undergoes when exposed to an alkali is truly remarkable. Instead of turning blue, like blue litmus paper would in the same circumstance, red litmus paper turns blue-purple. This unique color change is a definitive indicator of the presence of a base or alkali.
Chemical Reaction
The reason behind this color change lies in the chemical properties of the substances involved. When red litmus paper encounters an alkali, the hydroxide ions (OH-) present in the alkali react with the dye in the litmus paper. This reaction causes the red litmus paper to gain electrons and undergo a transformation that results in the blue-purple coloration.
Practical Applications of Red Litmus Paper
Red litmus paper’s ability to change color in the presence of acids and turn blue-purple in the presence of bases makes it an invaluable tool in various fields. In laboratories, red litmus paper is commonly used to quickly identify whether a given solution is acidic or basic. It is also utilized in educational settings to teach students about the properties of acids and bases in a hands-on manner.
In conclusion, when red litmus paper is placed in an alkali, it undergoes a fascinating color change from red to blue-purple. This transformation is a direct result of the chemical reaction between the acidic dye in the litmus paper and the hydroxide ions present in the alkali. Understanding the behavior of red litmus paper in different chemical environments provides valuable insights into the nature of acids and bases. So, next time you encounter red litmus paper, remember its magical color-changing abilities and the secrets it holds about the world of chemistry.
Litmus Test #chemistry
Frequently Asked Questions
What color does red litmus paper turn in an alkali solution?
Red litmus paper turns blue when it is placed in an alkali solution. This color change indicates the presence of a base or alkali due to the change in the pH level of the solution.
How can red litmus paper be used to test for the presence of an alkali?
To test for the presence of an alkali using red litmus paper, simply dip the red litmus paper into the solution being tested. If the red paper turns blue, it confirms the presence of an alkali in the solution.
What does the color change of red litmus paper indicate about the nature of the substance being tested?
When red litmus paper turns blue, it indicates that the substance being tested is basic or alkaline in nature. This color change is a reliable indicator of the presence of alkalis in the solution.
Final Thoughts
Red litmus paper turns blue when placed in an alkali. This color change is a clear and simple indicator of the presence of a base. It is a quick and easy way to determine the nature of a solution. Remember, if you’re ever unsure about the acidity or alkalinity of a substance, just test it with red litmus paper to see what color it turns when placed in an alkali!